Chiglitazar Sodium (trade name: Bilessglu®) is a novel mechanism new molecular entity drug exclusively discovered by the company. It is the world's first PPAR pan-agonist and belongs to the category of insulin sensitizers. Chiglitazar Sodium is an achievement of the National 863 Program and the “Significant New Drug Development” special project. It has been included in the textbooks Internal Medicine and the Guideline for the Prevention and Treatment of Diabetes Mellitus in China, and is expected to become a foundational drug for the comprehensive treatment of metabolic diseases.
Chiglitazar Sodium achieves a dynamic balance of glucose, lipid, and energy metabolism by moderately activating the three PPAR receptors, and improves metabolic diseases through multiple mechanisms such as glucose lowering and insulin sensitization, lipid regulation, anti-inflammation, and anti-fibrosis mechanisms. Multiple clinical trials have demonstrated the efficacy and safety of Chiglitazar Sodium in the treatment of type 2 diabetes and fatty liver disease.
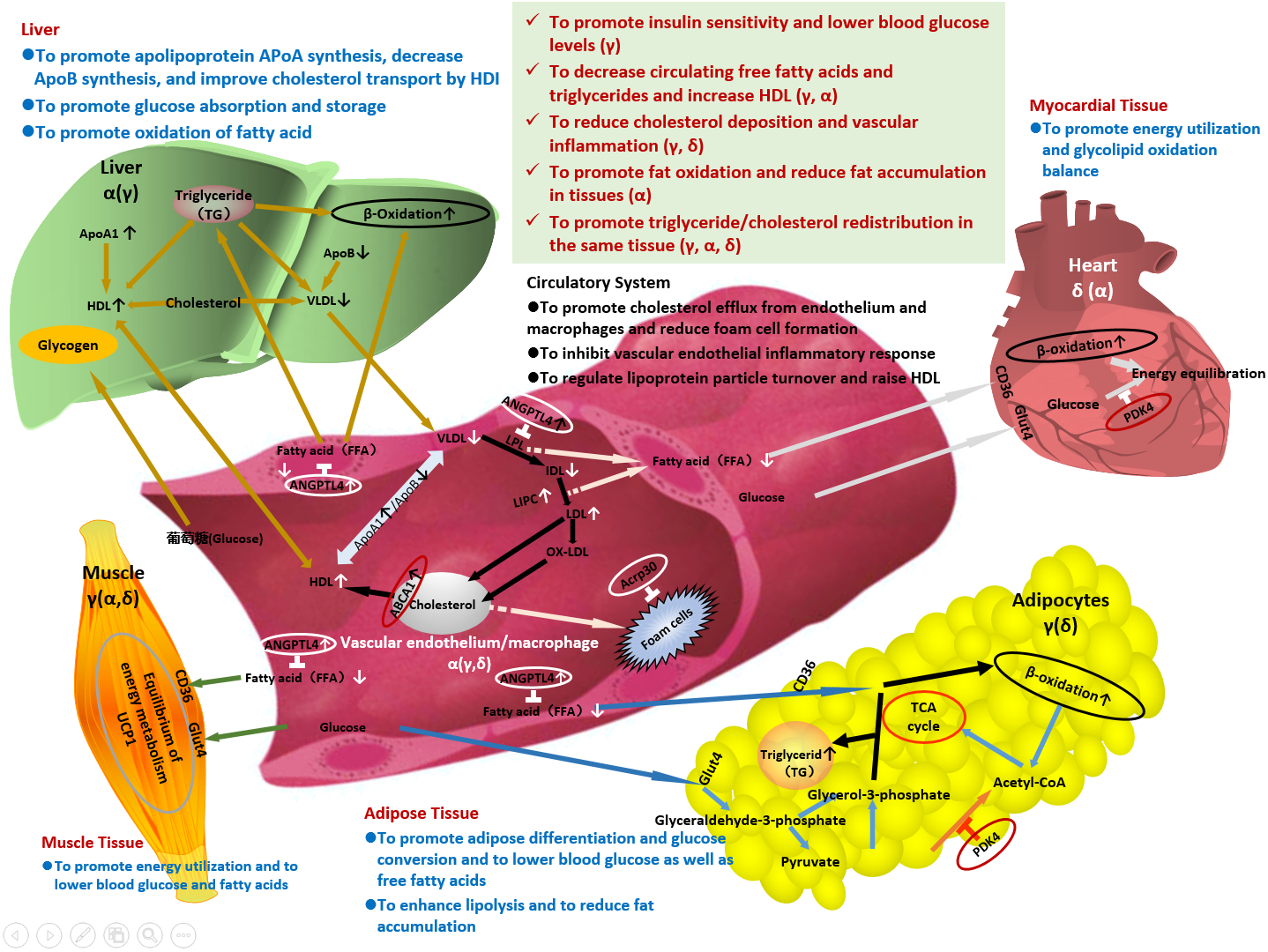
Figure Potential Mechanisms of Action of Chiglitazar Sodium
Commercialization Status in China
- In October 2021, Chiglitazar Sodium was approved for the treatment of type 2 diabetes (T2DM).
- In March 2023, Chiglitazar Sodium was included in the National Medical Insurance Drug List.
- In July 2024, Chiglitazar Sodium in combination with metformin was approved for the treatment of patients with type 2 diabetes mellitus inadequately controlled by metformin alone, providing a new option for combination therapy in type 2 diabetes patients.
- In November 2024, Chiglitazar Sodium was renewed at the original price in the National Medical Insurance Drug List.
Exploration of Other Indications
- In March 2024, a randomized, double-blind, placebo-controlled phase II clinical trial evaluating Chiglitazar Sodium monotherapy in the treatment of metabolic dysfunction-associated steatohepatitis (MASH) achieved its primary efficacy endpoints with favorable efficacy.
- In November 2024, the results of this study were presented at an oral presentation at the 75th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), delivered by Professor You Hong from Beijing Friendship Hospital, Capital Medical University, during the conference.
- In March 2025, at the Annual Meeting of the Asian Pacific Association for the Study of the Liver, Professor Zang Shufei from the Department of Endocrinology at Shanghai Fifth People's Hospital delivered an oral presentation on a new clinical study of Chiglitazar Sodium for the treatment of type 2 diabetes complicated with metabolic dysfunction-associated steatohepatitis. The study revealed that the pan-PPAR agonist Chiglitazar Sodium (Bilessglu®) 48 mg demonstrated favorable efficacy and safety in the treatment of high-risk populations with T2DM and MASH, offering new insights and options for clinical treatment.
- In June 2025, a real-world study on the effects of Chiglitazar Sodium on type 2 diabetes complicated with metabolic dysfunction-associated fatty liver disease was published in Diabetes & Metabolism, the official journal of the French Society for the Study of Diabetes (SFD). The study showed that Chiglitazar Sodium significantly reduced the controlled attenuation parameter (CAP) value, a key indicator of liver fatty in patients with metabolic dysfunction-associated steatotic liver disease and Type 2 diabetes mellitus, indicating its potential as a dual therapy for co-management of diabetes and liver disease.
- In August 2025, the phase II clinical trial results of Chiglitazar Sodium monotherapy for metabolic dysfunction-associated steatohepatitis were published in Hepatology, a top-tier international journal in the field of liver diseases. After 18 weeks of treatment, patients showed a 40% reduction in liver fat content, with more than 70% achieving normalization of liver enzyme levels.
Chiglitazar Sodium has been approved for the treatment of type 2 diabetes, accumulating extensive real-world medication experience. More than half of diabetes patients also suffer from metabolic dysfunction-associated fatty liver disease, and the majority of MASH patients seeking clinical treatment already have comorbid diabetes. Given that no drug has been approved for the treatment of fatty liver disease in China, there is a significant unmet clinical need. Based on existing clinical trial data and real-world medication evidence, the company will prioritize promoting Chiglitazar Sodium to benefit the broad population of patients with type 2 diabetes complicated with MASH/MAFLD. Meanwhile, the company will continue collaborating with investigators to strengthen the evidence for Chiglitazar Sodium in treating fatty liver disease across the entire disease course.

