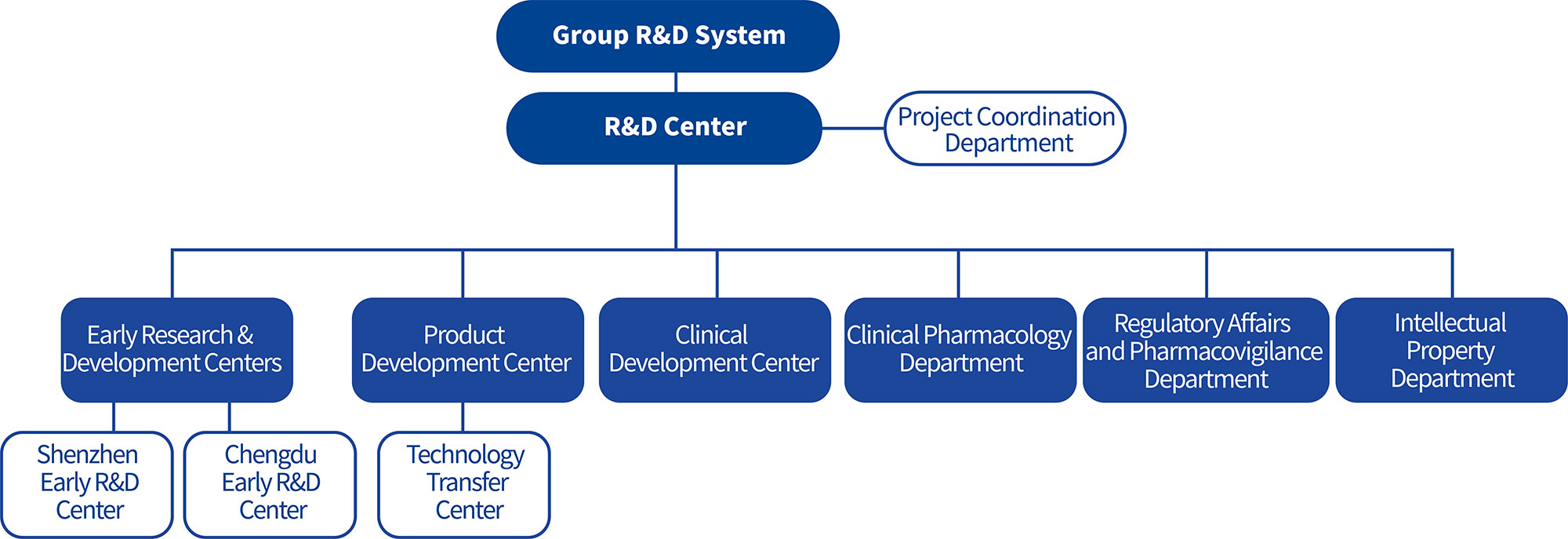
The development of pioneering innovative drugs involves the intersection and integration of multiple disciplines. Chipscreen Biosciences' core R&D management team consists of professionals with extensive experience in drug research, development, and management both from the United States and domestically, possessing rich expertise in international pharmaceutical/biotechnology enterprise management and R&D, and is well-versed in global drug regulatory technical requirements and patent strategies. The R&D team has professional/interdisciplinary backgrounds in medicine, pharmacy, chemistry, biology, and other related fields. The senior R&D talent team possesses expert knowledge in target validation, exploratory research, molecular simulation and design, high- output and high-content screening, and translational medicine research of biomarkers, with core capabilities in early-stage screening and evaluation of new drugs, clinical research and development, as well as research, pilot testing, quality control, and production from laboratory to commercialization. Talent, technology, and the R&D management system provide a foundation for the company's development of original innovative drugs.
Early Research & Development Centers
Chipscreen Biosciences is a pioneer in the field of original innovative drugs in China. As the core engine, the Early Research & Development Centers are grounded in science, committed to innovation, consistently focusing on unmet clinical needs, and continuously developing innovative drugs with significant clinical differentiation advantages and marketing potential. Through the integrated management of the Shenzhen and Chengdu Early Research & Development Centers across different fields, the company optimizes the allocation of human and R&D resources, accelerates product development and technological innovation, and provides strong support for the development and launch of new products. The Macro-Molecule Early Research & Development Center (Chipscreen Newway) further enriches the company's innovative drug modalities and therapeutic approaches.
Clinical Development Center
The clinical development strategies and practices for original innovative drugs with novel mechanisms and new indications are significantly different from those of fast-follower drugs and generics. Over the past two decades, Chipscreen Biosciences has explored and implemented strategies and models that align with the clinical development principles of original innovative drugs. The company has achieved successful cases spanning from early-stage clinical trials to registration clinical trials, from new drug marketing approval to post-marketing real-world observational studies, and from clinical research in China to international clinical development. A fully functional clinical development team has been established, with members specializing in clinical trial management, medical affairs, regulatory affairs, operations, data management, statistics, pharmacokinetics, and drug safety distributed across multiple cities nationwide, ensuring robust clinical development capabilities.
Clinical Pharmacology
Through clinical pharmacology research, a comprehensive understanding of the pharmacokinetic and pharmacodynamic characteristics of innovative drugs in humans is obtained, providing a scientific basis for the formulation of clinical development plans. This work is conducted in collaboration with teams from early R&D centers, clinical development, product development, and regulatory affairs to advance the regulatory approval and marketing applications of innovative drugs.
Product Development
Product development serves as a critical support for the smooth progression of clinical research and the industrialization of original innovative drugs, ensuring the controllable quality and safety of drugs intended for market launch. As the development process of original innovative drugs progresses and the understanding of the drugs deepens, pharmaceutical research must be continuously refined in conjunction with clinical studies and scale-up production. This includes process research, impurity studies, and quality research for innovative drugs to ensure their safety, efficacy, and controllable quality, thereby further guaranteeing the success rate of products in development. To date, Chipscreen Biosciences' product development system has accumulated extensive practical experience and critical capabilities for new product development through three original innovative drug projects: Chidamide, Chiglitazar Sodium, and Chiauranib.
Regulatory Affairs and Pharmacovigilance
Responsible for clinical trial applications, marketing applications, and the collection and reporting of drug safety information.
Intellectual Property
Through patent layout and maintenance related to product development and lifecycle management, legal protection is provided for the core interests of the company's innovative drugs, including their market value and social value.

