Chiauranib is a new molecular entity exclusively discovered by the company with global patent protection, featuring a novel mechanism as a triple-pathway tumor-targeting inhibitor. Chiauranib selectively inhibits protein kinase targets related to tumorigenesis and tumor development, including Aurora B, CSF1R, and VEGFR/PDGFR/c-Kit. It exerts multi-directional and broad-spectrum anti-tumor effects by inhibiting tumor cell proliferation, suppressing tumor angiogenesis, and regulating the tumor immune microenvironment, demonstrating favorable efficacy and safety in clinical trials of monotherapy for end-line tumor treatment.
With its highly selective inhibition of Aurora B/VEGFR/PDGFR/c-Kit/CSF1R targets, Chiauranib achieves comprehensive anti-tumor efficacy through three active mechanisms: inhibiting tumor cell mitosis/genomic stability, suppressing tumor angiogenesis, and modulating the tumor immune microenvironment. This multi-pathway mechanism results in superior pre-clinical pharmacodynamic activity compared to drugs with similar mechanisms and exhibits a favorable safety profile.
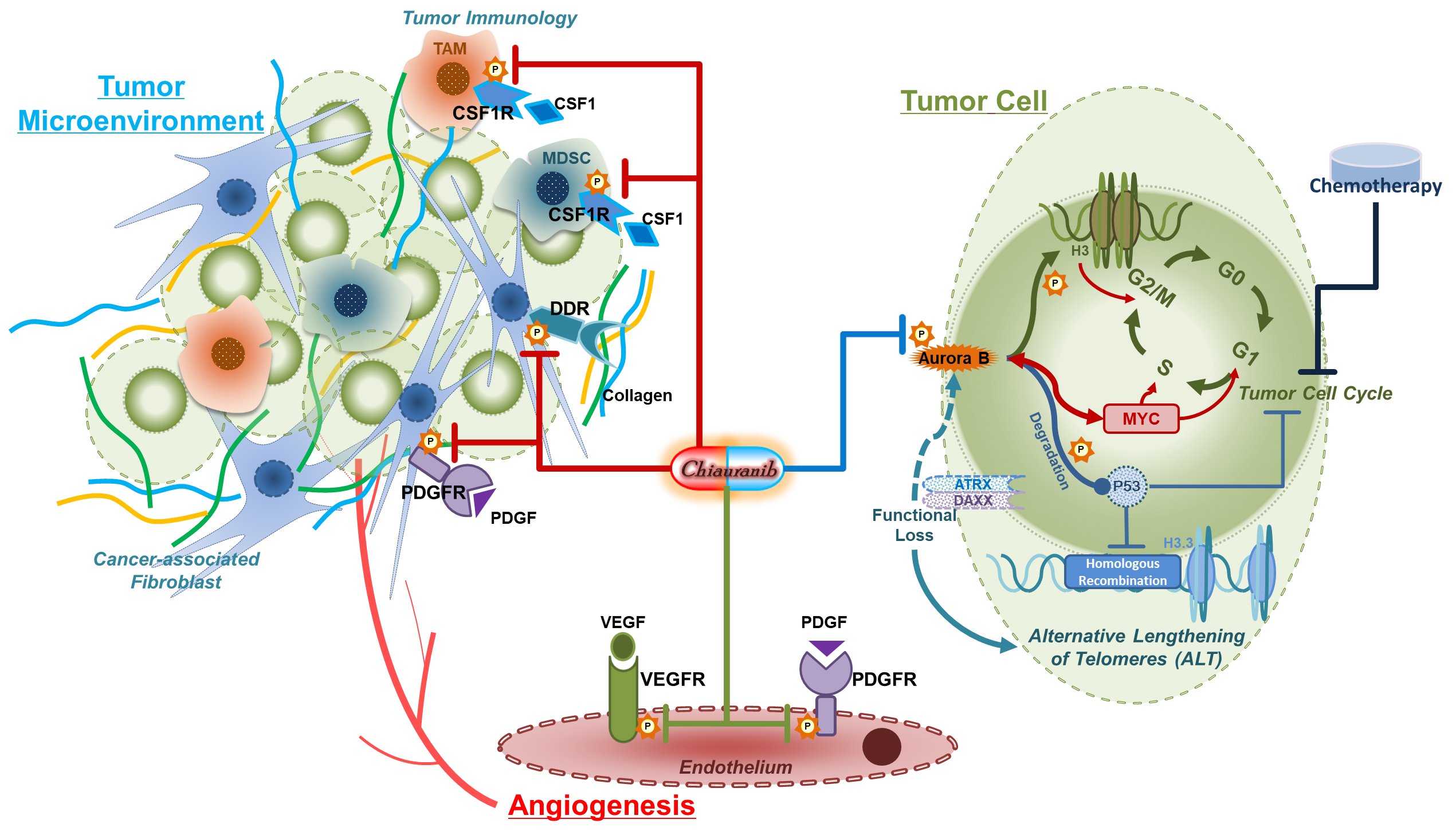
Figure Schematic Diagram of the Triple-Pathway Anti-Tumor Mechanism of Chiauranib
Global Development Status of Indications
- The phase II clinical trial evaluating Chiauranib in combination with albumin-bound paclitaxel and gemcitabine as first-line treatment for patients with locally advanced pancreatic ductal adenocarcinoma is progressing smoothly according to plan. As of the follow-up data on July 22, 2025, the 6-month PFS rate for the Chiauranib combination regimen as first-line therapy was approximately 80%, which is significantly better than the historical efficacy of chemotherapy in non-head-to-head comparisons (6-month PFS rate for standard first-line chemotherapy: 44%–56.4%), preliminarily demonstrating good antineoplastic activity and manageable safety.
- A randomized, double-blind, controlled, multicenter phase III clinical trial evaluating Chiauranib in combination with paclitaxel for the treatment of patients with platinum-refractory or platinum-resistant recurrent ovarian cancer is currently underway.
- All 12 clinical study sites for the phase Ib/II trial of Chiauranib in the United States have been operational, and the third dose level (65 mg) has completed escalation without any dose-limiting toxicity (DLT) observed. The FDA approved the exploration of higher doses under this protocol in March 2025.