Chidamide (trade name: Epidaza®) is a new molecular entity drug exclusively discovered by the company with a novel mechanism. It is the world's first subtype-selective histone deacetylase (HDAC) inhibitor and the first oral therapeutic drug approved for the treatment of peripheral T-cell lymphoma and advanced hormone receptor-positive breast cancer, belonging to the class of epigenetic modulators.
Chidamide acts on epigenetics-related targets—histone deacetylases (subtypes 1, 2, and 3 of Class I and subtype 10 of Class IIb). Histone deacetylases (HDACs) are a class of proteases that play a crucial role in chromosomal structural modification and gene expression regulation. As an HDAC inhibitor, Chidamide exerts its effects by inhibiting the biological activity of HDACs, thereby inducing changes in the gene expression of multiple signaling pathways involved in tumorigenesis (i.e., epigenetic changes).
Specifically, the general mechanisms of action of Chidamide primarily include: ① Directly inhibiting the tumor cell cycle and inducing apoptosis; ② Inducing and activating natural killer (NK) cell- and antigen-specific cytotoxic T lymphocyte (CTL)-mediated tumor killing effects; ③ Inhibiting phenotypic transformation of tumor cells and the pro-drug resistance/pro-metastasis activity in the tumor microenvironment.
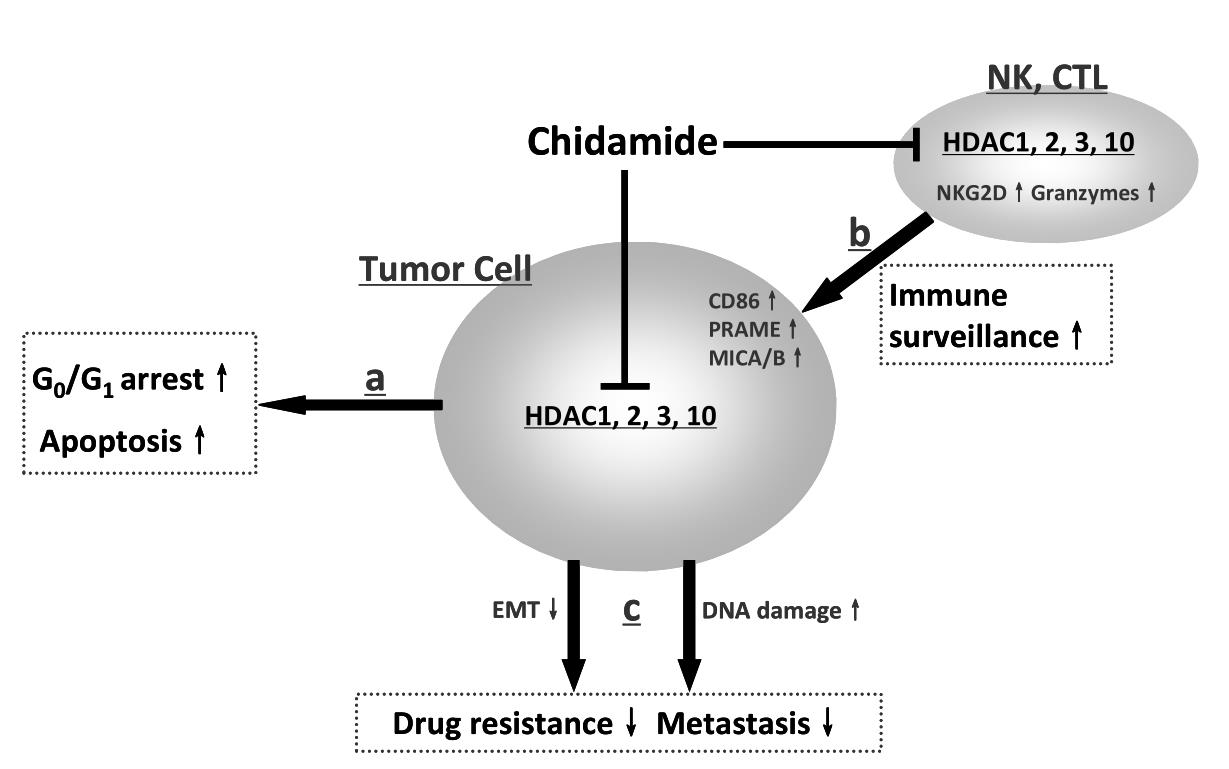
Figure: Molecular Anti-tumor Mechanisms of Action of Chidamide (Epidaza)
Commercialization Status
- In December 2014, it was approved in China for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma (PTCL) who had received at least one prior systemic chemotherapy, filling a gap in this treatment area in the country.
- In July 2017, it was included in the National Medical Insurance Drug List, benefiting more patients.
- In November 2019, it was approved in China in combination with an aromatase inhibitor for the treatment of postmenopausal patients with locally advanced or metastatic breast cancer that is hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative, and has relapsed or progressed after endocrine therapy. It was first confirmed that HDAC inhibitors in combination with other targeted drugs can effectively overcome tumor drug resistance, making it the world's first epigenetic modulator approved for the treatment of solid tumors.
- In June 2021, it was approved in Japan as a monotherapy for the treatment of adult patients with relapsed or refractory (R/R) T-cell leukemia (ATL).
- In December 2021, Chidamide received its second approval in Japan—as a monotherapy for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma.
- In March 2023, it was approved in Chinese Taiwan, for the treatment of postmenopausal patients with locally advanced or metastatic breast cancer that is hormone receptor-positive and human epidermal growth factor receptor 2 (HER2)-negative, and has relapsed or progressed after endocrine therapy (in combination with exemestane).
- In April 2024, it was approved in China in combination with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) for the treatment of treatment-naive patients with MYC- and BCL2-positive diffuse large B-cell lymphoma (DLBCL).
- In December 2024, the DLBCL indication of Chidamide was included for the first time in the National Medical Insurance Drug List, making it the only oral innovative drug for first-line treatment of DLBCL covered by national medical insurance.
Exploration of Other Indications Worldwide
- A randomized, double-blind, placebo-controlled, multicenter phase III clinical trial of Chidamide in combination with CHOP for the treatment of previously untreated peripheral T-cell lymphoma with T follicular helper phenotype (PTCL-TFH) is currently ongoing.
- A phase III clinical trial of Chidamide in combination with sintilimab and bevacizumab for the treatment of patients with advanced microsatellite stable or proficient mismatch repair (MSS/pMMR) colorectal cancer who have failed ≥2 lines of standard treatment is currently ongoing. A total of 430 subjects are planned to be enrolled, and as of June 30, 2025, 331 subjects have been enrolled.
- The randomized, double-blind, active-controlled phase III clinical trial evaluating Chidamide in combination with nivolumab as first-line treatment for advanced melanoma, conducted by the company's international partner HUYABIO International across 17 countries worldwide, has completed enrollment.
- Preclinical studies of NW001 (an epigenetic immune ADC candidate drug based on Chidamide) are currently underway.
At present, multiple clinical trials investigating Chidamide in combination with various cancer immunotherapies are being advanced both in China and internationally.

